
SYNAIRGEN
Approaching a Critical Phase
RETURN TO AIMZINE FRONT PAGE | September 2009
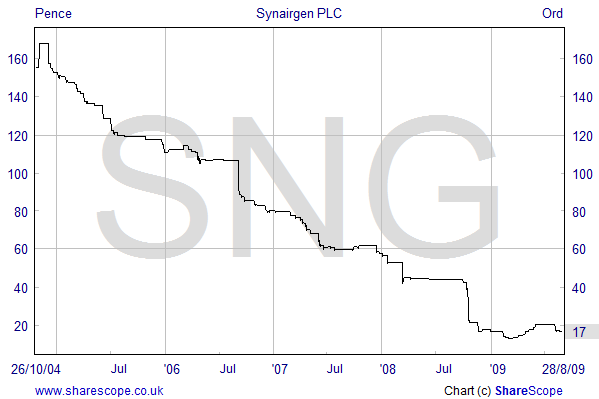
Code: SNG |
Sector: Pharmaceuticals |
| Price: 17P | Market Cap: £10 million |
| Locations: Southampton | Number of Staff: 22 (currently recruiting 4 more) |
In recent months our Aimzine Company features have focussed on small fast-growing AIM companies. However, this month we are looking at a very different proposition in Synairgen. Nonetheless, we believe that this Company provides a very exciting investment proposition. From the current level there is clear potential to make huge gains in a fairly short timescale. It should go without saying that such a proposition will involve considerable risk but, at least, progress to date is encouraging.
The Opportunity
When a healthy person contracts a common cold virus they are able to ‘throw off’ the cold in a few days. However, when someone suffering from Asthma or Chronic Obstructive Pulmonary Disease (COPD)** catches a cold, the symptoms can be much more severe and may lead to hospitalisation or, in some cases, even death.
Synairgen’s core activity involves research into the use of the protein interferon-beta (IFN-β) to reduce the impact that cold infections can have on COPD and Asthma sufferers. IFN-β is already used to treat millions of multiple sclerosis sufferers and Synairgen’s patents only deal with their method of use of the protein to treat these diseases.
I spoke to Synairgen’s Managing Director, Richard Marsden, about the prospects for the Company. Richard explained that there is potential for significant license payments for any successful treatment for exacerbations in Asthma and COPD. To give an idea of the importance of this field for the pharmaceutical industry: The annual revenue from the top 4 asthma drugs is currently around $12 million. Furthermore, in the USA alone there are thought to be over one million hospital admissions each year of Asthma and COPD patients suffering viral infections. Hence, a treatment that could significantly reduce hospital admissions could save billions of dollars each year as well as relieving a lot of illness and stress for the sufferers.
Synairgen expect to commence phase 2 testing of IFN-β in both Asthma and COPD patients early next year with results expected within 12 months. If these trials can demonstrate that IFN-β is a safe and effective treatment then the company anticipates that it will be able to out-license its technology to a large pharmaceutical company. With big pharma’s well publicised need for new pipeline products there is likely to be strong demand for a successful treatment programme. Synairgen’s broker, Matrix Corporate Capital, estimates that if the Phase 2 trials are successful then total milestone payments could be circa $200 million along with significant royalty payments.
 |
Richard Marsden using an I-neb nebuliser
**Chronic Obstructive Pulmonary Disease (COPD)
is an 'umbrella' term for people with chronic bronchitis,
emphysema, or both. With COPD the airflow to the lungs
is restricted (obstructed) COPD is commonly caused
by smoking. Symptoms include cough and breathlessness.
Successful Placing
Synairgen was spun-out from the University of Southampton's School of Medicine in 2003. The Company was floated on AIM in 2004, raising £9 million at the time of its Initial Public Offering. The shares have performed poorly since floatation in line with many other small companies in this sector. However, it is a credit to Synairgen that they were able to raise a further £6.35 million (gross), more than doubling the value of the Company, in May this year.
The May placing, at 17 pence per share, was supported by existing and new institutional investors and was achieved at only a small discount to the then current share price. Richard Marsden explained that the fund-raising had been signposted for some time and it had been relatively straightforward to arrange the fund raising. Approximately 60% of the funds raised came from existing investors.
It is encouraging to note that four Directors of the Company supported the funding although, even after this support, the total director holding is low for an AIM company at 4.33%. The largest shareholder is Lansdowne Partners at 19.99% and a total of 48% of the shares are deemed to be not in public hands. Southampton University retains a 6% shareholding and also has rights to royalty payments – details of which can be found in the 2004 IPO Prospectus, available here.
At the time of the fund raising Chairman, Simon Shaw, commented 'We are delighted to receive such strong support for this Fundraising, which will allow us to finance the planned Phase IIa studies of our exciting inhaled interferon beta product against virus-induced exacerbations in asthma and COPD. Thereafter, assuming the studies are successful, we should be well placed to seek out-licence terms for these two potentially very significant markets.’
they were able to raise
a further £6.35 million ...in
May this year
Synairgen at a glance |
+ Possibility of considerable gains |
+ Sufficient cash for next 2 years |
+ Positive early indications |
+ Double chance of success |
- Risk of unsuccessful Asthma trial |
- Risk of unsuccessful COPD trial |
- Slow approval and recruitment process |
- Significant setacks could cause funding problems |
Results and Forecasts
The Company is due to issue its results for the year to 30 June 2009 on 4 September. Assuming similar performance to the interims, we would expect that these will show a loss for the year of just under £3 million. The Company’s costs will increase over the coming year as it prepares for the Phase 2 trials but the Company anticipates having sufficient funds in place to complete these 2 trials.
Synairgen is relatively cheap to run as it is based in The School of Medicine at Southampton General Hospital where it is able use the existing infrastructure on a pay-for-use basis. Richard’s next appointment after speaking to Aimzine was to interview a potential recruit as part of their plan to increase numbers from 22 to 26 in preparation for the coming additional Phase 2 work.
Phase 2 Trials in 2010
Asthma is a human disease and as such cannot be fully replicated in trials with rodents. Instead the Company uses a biobank, which contains over 250 samples collected from human volunteers, to grow in vitro models of diseased lung. The expertise gained here would become an important part of any out-licensing deal to a large pharmaceutical company.
There are two separate trials scheduled to run in parallel starting in 2010, one for Asthma and one for COPD. The diseases are quite different; COPD is largely a result of cigarette smoking with some 25% of smokers developing the disease. Asthma is thought be caused by many factors, eg genetics, allergens, pollution and respiratory viruses. Importantly for Synairgen, the common cold is a problem for both asthmatic and COPD patients, the underlying biology is quite different for both diseases, and it is quite possible that inhaled interferon beta will work in one disease and not the other, thus Synairgen has ‘two rolls of the dice’, both markets having substantial potential. The two trials are scheduled to commence early in 2010 and are expected to take 12 months to complete. Prior to commencement of the trials the Company first needs to obtain the necessary approvals.
Synairgen reported in April that its Phase 1 trial in Asthmatic volunteers had shown increased anti-viral activity. At that time Professor Stephen Holgate (Non-executive Director and Co-Founder) stated that ‘this greatly increases our confidence of success in Phase 2 trials’. With this growing confidence the next 18 months will be an exciting period for Synairgen and its investors.
Like many AIM companies, Synairgen are involved in some fascinating technology but such technology can be difficult for investors to understand. The company has kindly agreed to produce an article for a future edition of Aimzine to explain more about Synairgen’s use of IFN-β and how the Company will be conducting Phase 2 trials of the product.
Bronchoscopy procedure
....thus Synairgen has
'two rolls of the dice'
Investment Considerations
The first thing that must be emphasised about Synairgen is that there are no guarantees of success. If next year’s trials are not successful there will be little residual value in the Company. Having said that there are good reasons why the chances of success are higher than for many other drug trials.
Interferon Beta is NOT a drug but a protein which is produced naturally by the body. With this in mind and the fact that IFN-β is already used to treat multiple sclerosis it would seem likely that the risk of the treatment not being tolerated in the trials is less than with many drug trials. Furthermore, as mentioned above, early indications from the laboratory tests and the Phase 1 trials are also encouraging. The success of the recent placing and the Director take up is, in our view, a further positive sign.
Another encouraging feature of Synairgen for investors is the relatively short timescale prior to a possible out-licensing deal. The Company are hoping that a deal can be completed soon after the completion of the phase 2 trials. The Company will thus avoid expensive, lengthy and not-without-risk Phase 3 trials.
The rewards for success in either (or both) Phase 2 trials could be considerable with 10 or even 20 fold gains seemingly quite possible for shareholders.
We are not qualified to comment on the likelihood of success for the IFN-β trials. There are however some things that appear to de-risk the programme. That Interferon Beta is already used in humans by injection provides some comfort, Interferon Beta itself is a recognised anti-viral, and the company has announced data that indicates they are successfully activating the antiviral biology in the lung in these early stage trials.
Ultimately risk investors must decide whether the price justifies the risk – we suspect that this Synairgen ‘bet’ is by far a better value one than many oil exploration and mining plays where small cap investor excitement can inflate prices far beyond fair value.
We will update readers on progress with Synairgen’s news items in our Featured Companies Update. If all seems to be going to plan we will be reading company announcements very carefully to look out for positive signs that out-licensing talks are progressing well.
patient using an inhaler with a spacer
Written by Michael Crockett, Aimzine
Copyright © Aimzine Ltd 2009
RETURN TO AIMZINE FRONT PAGE | September 2009
Discuss this article
|
||